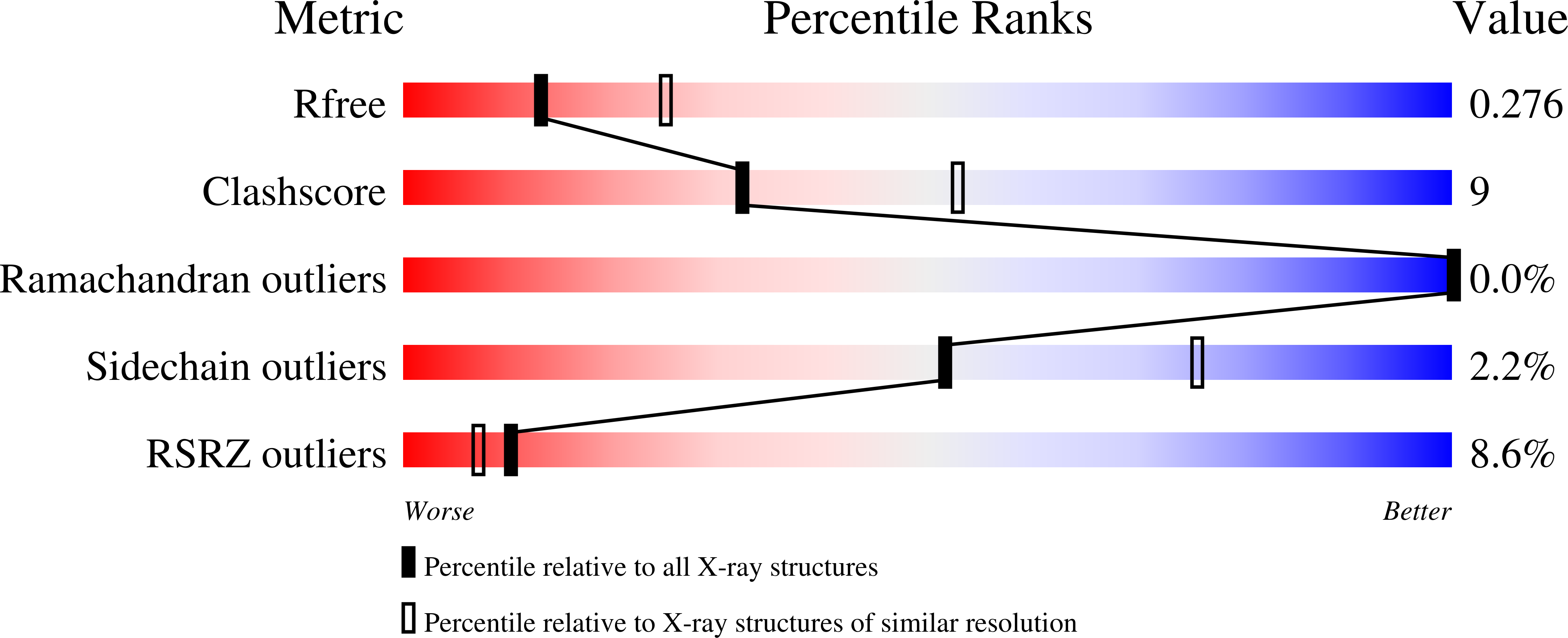
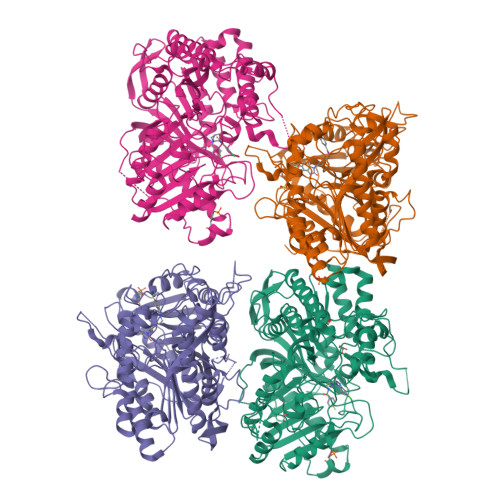
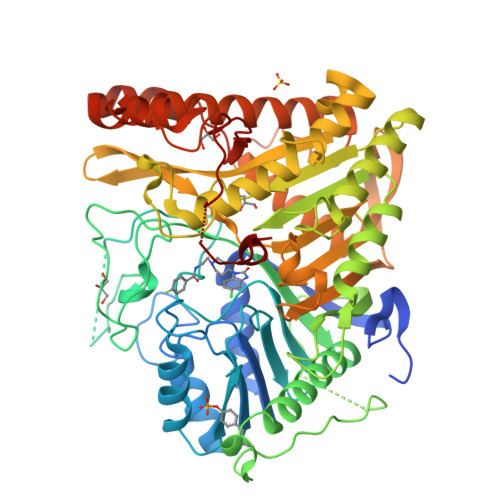
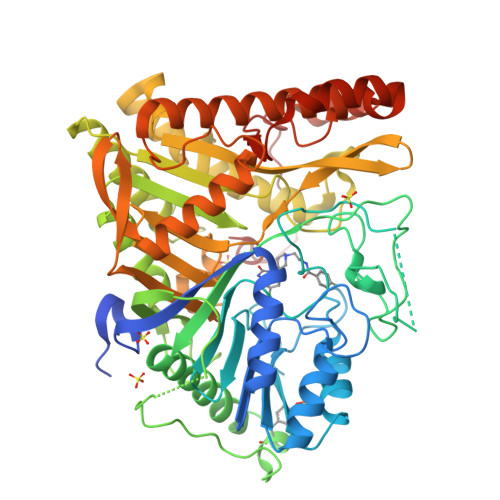
Human PLD structures enable drug design and characterization of isoenzyme selectivity.
Metrick, C.M., Peterson, E.A., Santoro, J.C., Enyedy, I.J., Murugan, P., Chen, T., Michelsen, K., Cullivan, M., Spilker, K.A., Kumar, P.R., May-Dracka, T.L., Chodaparambil, J.V.(2020) Nat Chem Biol 16: 391-399
- PubMed: 32042197
- DOI: https://doi.org/10.1038/s41589-019-0458-4
- Primary Citation of Related Structures:
6OHM, 6OHO, 6OHP, 6OHQ, 6OHR, 6OHS - PubMed Abstract:
Phospholipase D enzymes (PLDs) are ubiquitous phosphodiesterases that produce phosphatidic acid (PA), a key second messenger and biosynthetic building block. Although an orthologous bacterial Streptomyces sp. strain PMF PLD structure was solved two decades ago, the molecular basis underlying the functions of the human PLD enzymes (hPLD) remained unclear based on this structure due to the low homology between these sequences. Here, we describe the first crystal structures of hPLD1 and hPLD2 catalytic domains and identify novel structural elements and functional differences between the prokaryotic and eukaryotic enzymes. Furthermore, structure-based mutation studies and structures of inhibitor-hPLD complexes allowed us to elucidate the binding modes of dual and isoform-selective inhibitors, highlight key determinants of isoenzyme selectivity and provide a basis for further structure-based drug discovery and functional characterization of this therapeutically important superfamily of enzymes.
Organizational Affiliation:
Physical Biochemistry, Biotherapeutic and Medicinal Sciences, Biogen, Cambridge, MA, USA.